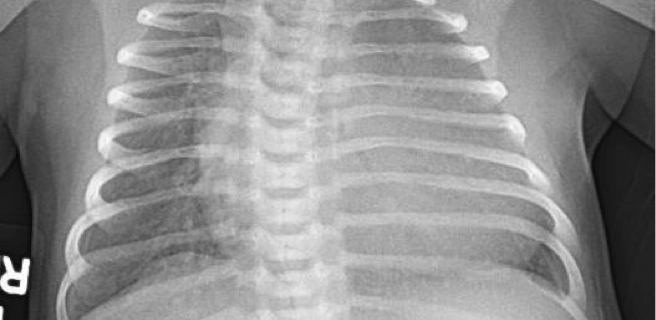
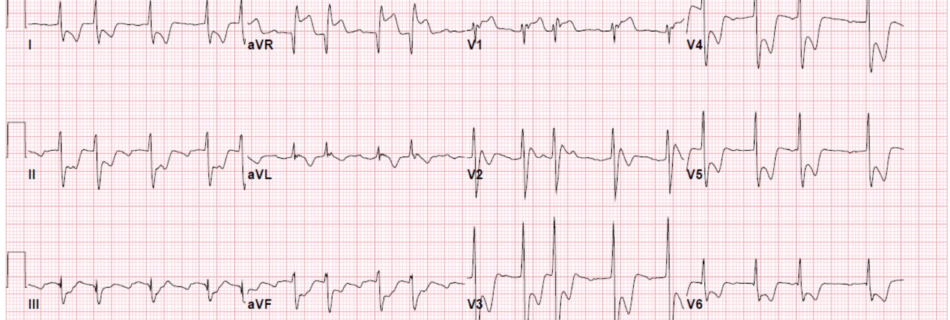
Case of the week COW #15
CC: Shortness of breath HPI: 7 day old female presents to the Emergency Dept. (ED) after being seen earlier in clinic. Mother is rom Nigeria and arrived to the U.S a few weeks prior to delivery. Prenatal care is unclear. Patient was delivered via C-Section at 39 weeks at another nearby hospital. Both mother and …