REBEL Core Cast – Basics of EM – Introduction
Written by EJ Wright, MD
This post first appeared on REBEL Cast
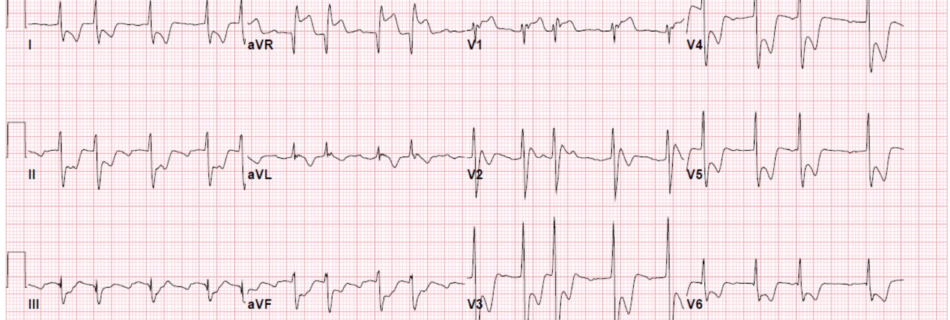
Welcome to the EMRA Basics of Emergency Medicine Podcast. I am your host EJ Wright, and the following series is an all encompassing approach to the most common chief complaints in the ED based on the well known EMRA Basics of Emergency Medicine, A Chief Complaint-Based Guide. Each cast will highlight myself and a guest attending physician as we take new learners through the differentials, red flags, physical exam findings, and a sample presentation that you need to know to treat patients in the ED.