Written by Christopher Hart, DO
This post first appeared on REBEL EM [Link is here]
Introduction: Acetaminophen, N-acetyl-p-aminophenol, or APAP, is one of the most commonly used medications worldwide. While it is generally safely used, overdose can result in the development of liver failure due to APAP’s hepatotoxic metabolites. Hepatic necrosis is preventable in overdose with timely administration of N-acetyl-cysteine (NAC) which restores glutathione reserves, allowing for safe excretion of these hepatotoxic metabolites. NAC use is based on plotting APAP levels, on the Rumack-Matthew nomogram:
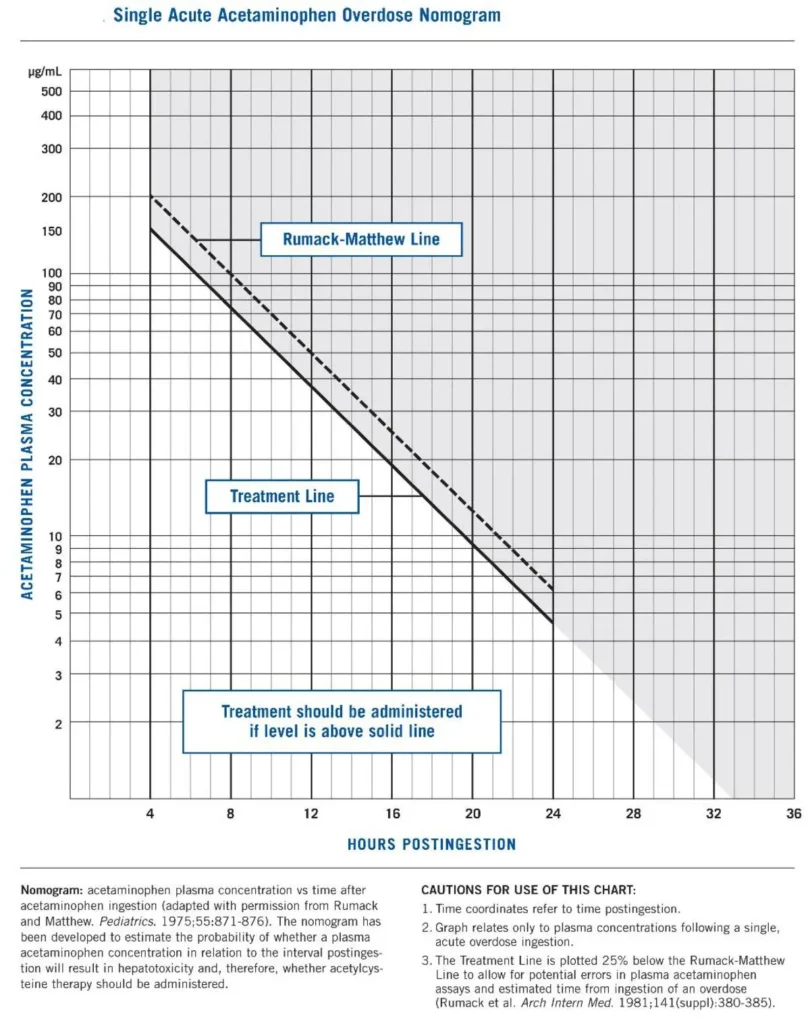
Image from Wikipedia [Link is HERE]
Intravenous NAC therapy includes:
- An initial dose of 150 mg/kg over 1 hour,
- A second dose of 50 mg/kg over 4 hours,
- And a final infusion of 100 mg/kg over 16 hours.
However, it is unclear if this dosing regimen is adequate for the treatment of massive overdoses (>32g or concentrations >300 mcg/mL). Before the advent of IV NAC in 2002, oral NAC was the standard. Though IV and oral NAC loading doses were similar (140-150 mg/kg), the oral NAC regimen provided an additional 70 mg/kg every 4 hours for 17 doses, while the IV NAC regimen provided an additional 12.5 mg/kg/h for 4 hours, followed by 6.25 mg/kg/hr for the next 16 hours.
When oral NAC was the sole option, there were no reported cases of liver failure or death when clinicians treated patients within 8 hours. However, there have been several cases of liver failure in acetaminophen overdoses with the current IV dosage recommendations. In addition, the risk of hepatotoxicity substantially increases with APAP concentrations over 300mcg/mL.
Clinical Question: Will increasing the third dose of IV NAC decrease the risk of hepatotoxicity in massive APAP overdose?
Article: Hendrickson RG. What is the most appropriate dose of N-acetylcysteine after a massive acetaminophen overdose? Clin Toxicol (Phila). 2019;57(8):686-691. PMID: 30777470
Type: Narrative Review
Background:
Cairney et al. showed that despite treatment with NAC, in massive overdoses, patients with APAP concentrations above the 300 mcg/mL and 500 mcg/mL line on the Rumack-Matthew nomogram developed hepatotoxicity when compared with lower APAP concentrations. (Cairney 2016) Similarly, Marks et al. retrospectively showed increased rates of hepatotoxicity and coagulopathy at APAP concentrations above the 300- and 500-lines. (Marks 2017)
Chiew et al. attempted to prevent hepatotoxicity in massive APAP overdoses by doubling the rate of NAC infusion over the last 16 hours, giving patients 12.5 mg/kg/h instead of the standard 6.25 mg/kg/h. (Chiew 2017) There were no cases of hepatotoxicity when treating APAP concentrations between 300 mcg/mL and 450 mcg/mL with the double infusion rate. Still, they did not prevent hepatotoxicity in patients with an APAP concentration over 450 mcg/mL.
These increased doses are based on the hypothesis that higher doses of NAC are required proportionate to larger ingestions and higher APAP levels.
Increased Dosing:
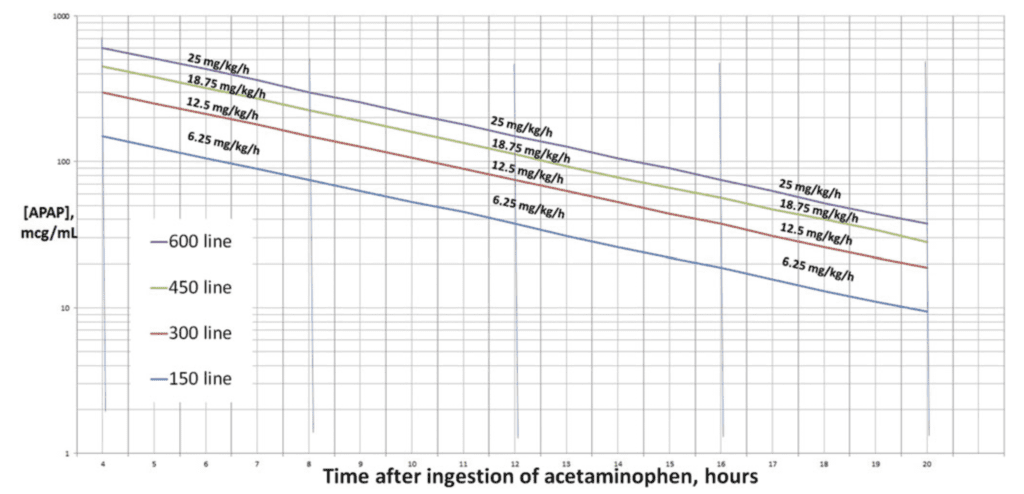
The Rumack-Matthew nomogram suggests a final NAC infusion rate of 6.25 mg/kg/h to treat a 16 g acetaminophen overdose. Theoretically, NAC should be supplied at a 1:1 molar ratio with NAPQI, one of the major hepatotoxic metabolites. The paper proposes, a final infusion rate of 12.5 mg/kg/h for a 32 g overdose, and a final infusion rate of 18.75 mg/kg/h for a 48 g overdose.
This dosing is based on several assumptions: glutathione stores are at less than 30% of normal, overdose levels peak at 4 hours, and no further absorption occurs after the initial overdose
- Range of final hourly infusion= [APAP]/32 to [APAP]/16.
- Thus, with an [APAP] of 300 mcg/mL, the infusion rate should be 9-18 mg/kg/h.
- This argues for an increase to 12.5 mg/kg/h infusion for [APAP] >300,
- And 18.75 mg/kg/hr for [APAP] >450.
How to Deliver:
- The protocol suggested in this study maintains the standard rates for the first and second doses of NAC but increases the final 16-hour dose.
- This can be achieved by either increasing the rate by infusing over a shorter time or increasing the concentration of NAC in the final infusion.
- Both of these options pose a risk to the patient: increasing the rate can result in fluid overload and increasing the concentration risks hyperosmolarity.
- Additionally, with increasing rate or concentration, there is also a risk of anaphylactoid reactions.
Strengths:
- Based on a few small case series, this descriptive study provides some evidence of massive acetaminophen overdoses being treated successfully without hepatotoxicity with an increased final infusion of NAC.
- This study lays the groundwork and develops a plan to treat massive overdoses to prevent hepatotoxicity.
Limitations:
- Clinical data is lacking and the studies presented have few participants, thus making it difficult to establish hard clinical evidence-based recommendations.
- In the Chiew study, some patients who developed hepatotoxicity were treated outside of the time window, including some at 13 hours. Therefore, delayed treatment, rather than dose alone, could be the actual cause of hepatotoxicity in these patients.
- The author makes nine assumptions to justify increasing the final infusion of NAC, including that 25-50% of the APAP will become NAPQI and that no further absorption of acetaminophen will occur after the initial overdose.
- The enzyme that produces NAPQI, CYP2E1, is an inducible enzyme and thus, with other substances such as ethanol, can be induced and possibly create more NAPQI and liver injury on its own.
- The effectiveness of NAC depends on the patient having adequate glutamic acid and glycine stores, which are required in the pathway to synthesize glutathione and detoxify NAPQI. (Athersuch 2018; Heard 2008)
Discussion:
- Based on the evidence, there is an argument for increasing the current doses of NAC based on the level of serum acetaminophen. Considering that this study focused mainly on patients above the 300-line, this would be a reasonable level at which to start increasing NAC dosing.
- The few studies cited represent limited data on this new regimen but show promising results. Doubling the final infusion to 12.5 mg/kg/h for APAP concentrations over 300 mcg/mL has been shown to decrease cases of hepatotoxicity. Continuing to increase the dosage beyond the 450 mcg/mL concentration to 18.75 mg/kg/h and the dosage above the 600 mcg/mL line to 25 mg/kg/h seems reasonable.
- However, further study is needed to determine if even higher doses than those suggested in the paper would benefit patients with these levels of massive overdose. The incidence of hepatotoxicity in patients at these levels above 300 mcg/mL treated with higher NAC doses was very low. Further studies are needed to precisely determine the appropriate dose of NAC for these cases.
- As always, consult your local expert toxicologists to help guide management decisions.
Author’s Conclusions: “Although toxicologists and poison centers generally agree that massive acetaminophen overdoses warrant increased or intensified dosing of IV NAC, there is little consensus on how to do this. Extrapolation from prior data suggests that the final NAC infusion rate should be 12.5 mg/kg/h for patients with APAP concentrations above the 300 mcg/mL line or with ingestions of 32 grams. Similarly, the 450 mcg/mL line (or 48 g ingestion) and the 600 mcg/mL line (or 64 g ingestion) may require final infusion rates of 18.75 mg/kg/h and 25 mg/kg/h, respectively. Future prospective research should evaluate this approach and should further refine the thresholds and dosing rates.”
Our Conclusions: The data presented highlights the potential for hepatotoxicity in patients with massive acetaminophen overdose despite timely treatment with NAC. As is typical for many toxicology interventions, there may never be a randomized controlled trial. However, the benefits of increasing the dose of NAC appear to outweigh the risks.
Potential to Impact Current Practice:
Increased doses of NAC are likely required to avoid hepatotoxicity in massive acetaminophen overdoses above 300 mcg/mL line or with ingestions above 32 grams.
Bottom Line:
- Massive acetaminophen overdoses (above 300 mcg/mL) may lead to hepatotoxicity despite early treatment with NAC.
- One standard dosing regimen may not be adequate for all patients
- Increased doses of NAC may be warranted for massive acetaminophen overdose.
References:
- Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics. 1975 Jun;55(6):871-6. PMID: 1134886
- Cairney DG, Beckwith HK, Al-Hourani K, Eddleston M, Bateman DN, Dear JW. Plasma paracetamol concentration at hospital presentation has a dose-dependent relationship with liver injury despite prompt treatment with intravenous acetylcysteine. Clin Toxicol (Phila). 2016 Jun;54(5):405-10. PMID: 27108714
- Marks DJB, Dargan PI, Archer JRH, et al. Outcomes from massive paracetamol overdose: a retrospective observational study. Br J Clin Pharmacol. 2017;83:1263–1272. PMID: 28002875
- Chiew AL, Isbister GK, Kirby KA, et al. Massive paracetamol overdose: an observational study on the effect of activated charcoal and increased acetylcysteine dose (ATOM 2). Clin Toxicol. 2017; 55:1055–1065. PMID: 28644687
- Athersuch TJ, Antoine DJ, Boobis AR, et al. Paracetamol metabolism, hepatotoxicity, biomarkers and therapeutic interventions: a perspective. Toxicol Res (Camb). 2018;7(3):347-357. Published 2018 Mar 6. PMID: 30090586
- Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359(3):285-292. PMID: 18635433
Guest Post By:

Christopher Hart, DO
PGY-1, Emergency Medicine Resident
Saint Joseph’s University Medical Center, Paterson, New Jersey
Twitter: @chris_hart10

Steven Hochman, MD FACEP
Associate Professor of Emergency Medicine
Saint Joseph’s University Medical Center, Paterson, New Jersey
Twitter: @hochmast

Marco Propersi, DO FAAEM
Assistant Professor of Emergency Medicine
Vassar Brothers Medical Center, Poughkeepsie, New York
Twitter: @marco_propersi
Post-Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)
